Our lab is uncovering how complex patterns of activity across populations of neurons are decoded to guide behavior in health and disease. Our research uses novel techniques to identify and restore failures in brain activity that lead to memory impairment with a special focus on observing and interrogating the hippocampal system during learning and memory. Using non-invasive approaches, we are translating these discoveries from rodents to develop radically new ways to treat diseases that affect memory in humans, like Alzheimer’s. Here are a few of the current projects in the lab:
Decoding Memory in Health and Disease
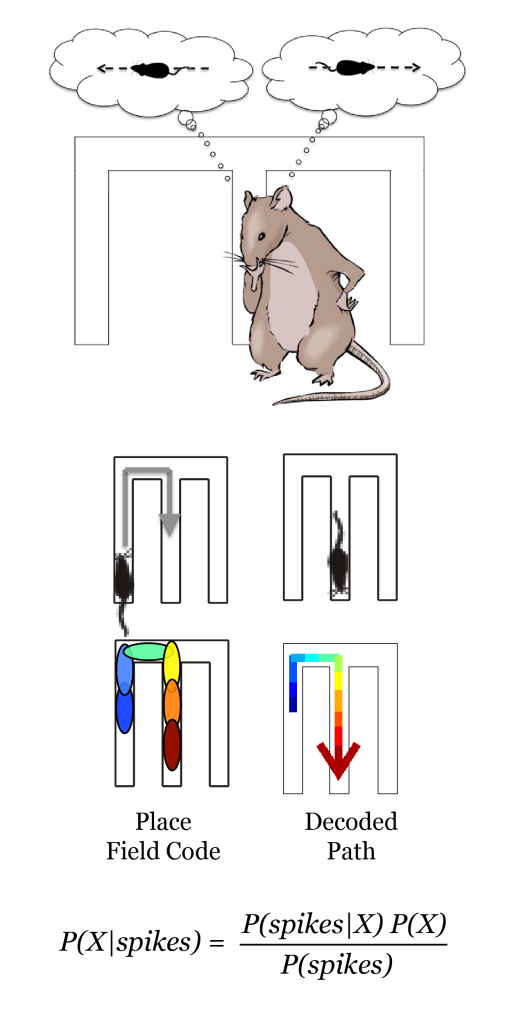
Memory is thought to require repeated reactivation of neural activity that represents learned behaviors (replay). This repeated replay of activity on short timescales likely strengthens connections between the active neurons and thus makes it possible to recall the learned behavior. Replay increases during learning and predicts correct decisions in a spatial task. If this replay activity is lacking, animals are less likely to choose the correct path to a reward. My lab has found replay in mouse models of AD. To achieve this, my lab records neural activity from many neurons as mice navigate a virtual reality environment (Iaccarino*, Singer* et al. 2016, Lee et al. 2017, Singer et al. 2017, Kolb et al. 2017) which enables a variety of methods for recording and manipulating neural activity during behavior. In mouse models of Alzheimer’s disease, we have found drastically impaired replay activity of prior experiences and in synaptic strength during SWRs (Prince et al., under review). These findings connect multiple levels of Alzheimer’s dysfunction from synaptic deficits to neural activity and memory processes. We are now determining how these deficits in neural activity lead to memory failures (Jeong et al. in prep), developing new tool to analyze this activity (Zhang et al. 2019), and discovering how neural activity that represents goal and task information fails in Alzheimer’s model mice (Zhang et al. in submission).
Relevant publications:
Sub-second Dynamics of Theta-Gamma Coupling in Hippocampal CA1.
Zhang L, J Lee , CJ Rozell, AC Singer (2019)
eLife
https://elifesciences.org/articles/44320
Gamma oscillations attenuate amyloid pathology and trigger a distinct microglia response in a mouse model of Alzheimer’s disease. Iaccarino HF*, Singer AC*, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, Mathys H, Seo J, Kritskiy O, Abdurrob F, Adaikkan C, Canter RG, Rueda R, Brown EN, Boyden ES, Tsai L-H (2016)
Nature
doi: 10.1038/nature20587. PMID: 27929004.
Published with News and Views. Recommended on Faculty of 1000.
Hippocampal SWR activity predicts correct decisions during the initial learning of an alternation task. Singer AC, MF Carr, MP Karlsson, LM Frank (2013)
Neuron
doi: 10.1016/j.neuron.2013.01.027. PMID: 23522050.
Featured article.
Experience dependent development of coordinated hippocampal spatial activity representing the similarity of related locations. Singer AC, MP Karlsson, AR Nathe, MF Carr, LM Frank (2010)
Journal of Neuroscience
doi: 10.1523/JNEUROSCI.0926-10.2010. PMID: 20810880
Rewarded outcomes enhance reactivation of experience in the hippocampus. Singer AC and LM Frank (2009)
Neuron
doi: 10.1016/j.neuron.2009.11.016. PMID: 20064396.
Cover article.
Novel Neural Stimulation to Control of Immune Cells and Pathology
 Recently we and collaborators discovered a surprising new way to manipulate microglia, the brain’s primary immune cells which play key roles in neurological and psychiatric disease as well as in normal brain functions like learning. While microglia are well known to affect neural function, little is known about how neural activity affects these immune cells and brain immune function generally. Inspired by neural activity deficits we found in animal models of Alzheimer’s disease, we found that driving gamma oscillations (40Hz) mobilized microglia to remove amyloid beta, a protein whose aggregation is thought to initiate neurotoxic events in Alzheimer’s disease (Iaccarino*, Singer* et al. 2016). Specifically, driving gamma recruited microglia to increase engulfment of beta amyloid, resulting in 40% reduction in beta amyloid. After initially using invasive optogenetics to drive gamma, we then discovered that these effects could be achieved with simple flickering lights and sounds at gamma frequency (similar to a fast strobe light and beeping). This simple and non-invasive sensory stimulation drove gamma oscillations in memory circuits, recruited immune cells, and rescued memory behavior (Singer et al. 2018, Martorell*, Paulson* et al. 2019, Paulson et al.in prep). Using this sensory stimulation, we have discovered a rapid and transient cascade of intracellular and extracellular biochemical immune signals by which driving gamma recruits immune cells (Garza et al. 2020, Garza et al. in prep). Furthermore, different frequencies of stimulation cause different effects on immune signals. Thus, we have developed a tool to rapidly increase or decrease genes and proteins that control brain immune function. Now we are using this flexible approach to manipulate neuroimmune function in other diseases (Franklin et al., in prep).
Recently we and collaborators discovered a surprising new way to manipulate microglia, the brain’s primary immune cells which play key roles in neurological and psychiatric disease as well as in normal brain functions like learning. While microglia are well known to affect neural function, little is known about how neural activity affects these immune cells and brain immune function generally. Inspired by neural activity deficits we found in animal models of Alzheimer’s disease, we found that driving gamma oscillations (40Hz) mobilized microglia to remove amyloid beta, a protein whose aggregation is thought to initiate neurotoxic events in Alzheimer’s disease (Iaccarino*, Singer* et al. 2016). Specifically, driving gamma recruited microglia to increase engulfment of beta amyloid, resulting in 40% reduction in beta amyloid. After initially using invasive optogenetics to drive gamma, we then discovered that these effects could be achieved with simple flickering lights and sounds at gamma frequency (similar to a fast strobe light and beeping). This simple and non-invasive sensory stimulation drove gamma oscillations in memory circuits, recruited immune cells, and rescued memory behavior (Singer et al. 2018, Martorell*, Paulson* et al. 2019, Paulson et al.in prep). Using this sensory stimulation, we have discovered a rapid and transient cascade of intracellular and extracellular biochemical immune signals by which driving gamma recruits immune cells (Garza et al. 2020, Garza et al. in prep). Furthermore, different frequencies of stimulation cause different effects on immune signals. Thus, we have developed a tool to rapidly increase or decrease genes and proteins that control brain immune function. Now we are using this flexible approach to manipulate neuroimmune function in other diseases (Franklin et al., in prep).
Relevant publications:
GarzaK, Zhang L, Boron B, Attokaren M,Wood L++, Singer AC++ (2020) “Gamma visual stimulation induces a neuroimmune signaling profile distinct from acute neuroinflammation.”
Journal of Neuroscience
https://doi.org/10.1523/JNEUROSCI.1511-19.2019
Multi-sensory gamma stimulation ameliorates Alzheimer’s-associated pathology and improves cognition. Martorell AJ*, AL Paulson*, H-J Suk, F Abdurrob, GT Drummond, W Guan, JZ Young, DN-W Kim, O Kritskiy, SJ Baker, V Mangena, SM Prince, EN Brown, KC Chung, ES Boyden, AC Singer,L-H Tsai
Cell
https://www.cell.com/cell/fulltext/S0092-8674(19)30163-1
Non-invasive 40 Hz light flicker to reduce amyloid load and recruit microglia. Singer AC++, AJ Martorell, JM Douglas, F Abdurrob, M Attokaren, J Tipton, H Mathys, C Adaikkan, and L-H Tsai++ (2018)
Nature Protocols.
https://www.nature.com/articles/s41596-018-0021-x
Gamma oscillations attenuate amyloid pathology and trigger a distinct microglia response in a mouse model of Alzheimer’s disease. Iaccarino HF*, Singer AC*, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, Mathys H, Seo J, Kritskiy O, Abdurrob F, Adaikkan C, Canter RG, Rueda R, Brown EN, Boyden ES, Tsai L-H (2016)
Nature.
doi: 10.1038/nature20587. PMID: 27929004.
Published with News and Views. Recommended on Faculty of 1000.
Principles of Designing Interpretable Optogenetic Behavior Experiments.
BD Allen*, Singer AC*, ES Boyden (2015)
Learning and Memory.
doi: 10.1101/lm.038026.114. PMID: 25787711.
Invited review.
Translation to Humans

Using non-invasive sensory stimulation, we are translating our results from mice to humans to determine if this stimulation reaches memory circuits and manipulates neuroimmune function in humans. We recently completed an initial feasibility trial of gamma sensory stimulation in patients with prodromal Alzheimer’s disease in collaboration with Drs. Allan Levey and Jim Lah at the Emory Brain Health Center (He et al. under review). We found excellent safety, tolerance, and adherence to gamma sensory Flicker and preliminary evidence that this stimulation affects the human neuroimmune system. Furthermore, we have found preliminary evidence that this stimulation entrains neural activity in human memory circuits using intracranial recordings in epilepsy patients (Blanpain et al., in prep). These discoveries will reveal new ways to non-invasively manipulate the human neuroimmune system and non-invasively target memory circuits in humans.