Research
Decoding Memory in Health and Disease
We recently discovered a new function for interneurons in gating and unlocking the formation of important memories. Inhibitory interneurons are typically thought of as having little spatially specific neural activity. In contrast, we find interneurons have coordinated seconds-long decreases in activity approaching goal location (Jeong*, Zheng* et al. 2025). Using closed loop optogenetic stimulation, we find that this decrease in inhibition is vital to rapid spatial learning and neural mechanisms of spatial memory. Decreases in inhibition around goals enable activity called “reactivation” in which neural activity representing past and future experience is replayed on the short timescales required for plasticity. Extensive research has shown that reactivation of prior experiences occurs during sharp wave ripples (SWRs) and this activity is essential for memory processes. Because interneurons are vulnerable to pathology, this discovery suggest how such interneuron vulnerability contributes to memory impairment. Indeed, we have found deficits in SWRs and reactivation in mouse models of Alzheimer’s (Prince et al., 2019, Iaccarino*, Singer* et al. 2016). Furthermore, we have discovered how neural activity that represents goal and task information fails in Alzheimer’s model mice (Zhang et al. 2022). We are now discovering how multiple cells and region work together to adapt navigation plans in dynamic environments (Prince et al. 2025).
Relevant Publications
- Goal specific hippocampal inhibition gates learning.
Jeong N+, Zheng X+, Paulson AL, Prince SM, Nguyen VP, Thomas SR, Gilpin CE, Goodson MC, Singer AC (2025)
Nature
https://doi.org/10.1038/s41586-025-08868-5
- New information triggers prospective codes to adapt for flexible navigation.
Prince SM, Yassine TA, Katragadda N, Roberts TC, Singer AC (2025)
Nature Communications
https://doi.org/10.1038/s41467-025-60122-8
- Learning from inhibition: Functional roles of hippocampal inhibition in spatial and episodic learning and memory. Jeong N, Singer AC (2022)
Current Opinion in Neurobiology
https://doi.org/10.1016/j.conb.2022.102604
- Goal discrimination in hippocampal non-place cells when place information is ambiguous. Zhang, L, Prince SM, Paulson AL, Singer AC (2022)
Proceedings of the National Academy of Sciences
https://doi.org/10.1073/pnas.2107337119
- Alzheimer’s pathology causes impaired inhibitory connections and reactivation of place codes during spatial navigation. Prince SM, Paulson AL, Jeong N, Amigues S, Singer AC (2021)
Cell Reports
https://doi.org/10.1016/j.celrep.2021.109008
Cover article.
- Sub-second Dynamics of Theta-Gamma Coupling in Hippocampal CA1.
Zhang L, J Lee , CJ Rozell, AC Singer (2019)
eLife
https://elifesciences.org/articles/44320
- Gamma oscillations attenuate amyloid pathology and trigger a distinct microglia response in a mouse model of Alzheimer’s disease. Iaccarino HF*, Singer AC*, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, Mathys H, Seo J, Kritskiy O, Abdurrob F, Adaikkan C, Canter RG, Rueda R, Brown EN, Boyden ES, Tsai L-H (2016)
Nature
doi: 10.1038/nature20587. PMID: 27929004.
Published with News and Views. Recommended on Faculty of 1000.
- Hippocampal SWR activity predicts correct decisions during the initial learning of an alternation task. Singer AC, MF Carr, MP Karlsson, LM Frank (2013)
Neuron
doi: 10.1016/j.neuron.2013.01.027. PMID: 23522050.
Featured article.
- Experience dependent development of coordinated hippocampal spatial activity representing the similarity of related locations. Singer AC, MP Karlsson, AR Nathe, MF Carr, LM Frank (2010)
Journal of Neuroscience
doi: 10.1523/JNEUROSCI.0926-10.2010. PMID: 20810880
- Rewarded outcomes enhance reactivation of experience in the hippocampus. Singer AC and LM Frank (2009)
Neuron
doi: 10.1016/j.neuron.2009.11.016. PMID: 20064396.
Cover article.
Novel Neural Stimulation to Control of Immune Cells and Pathology
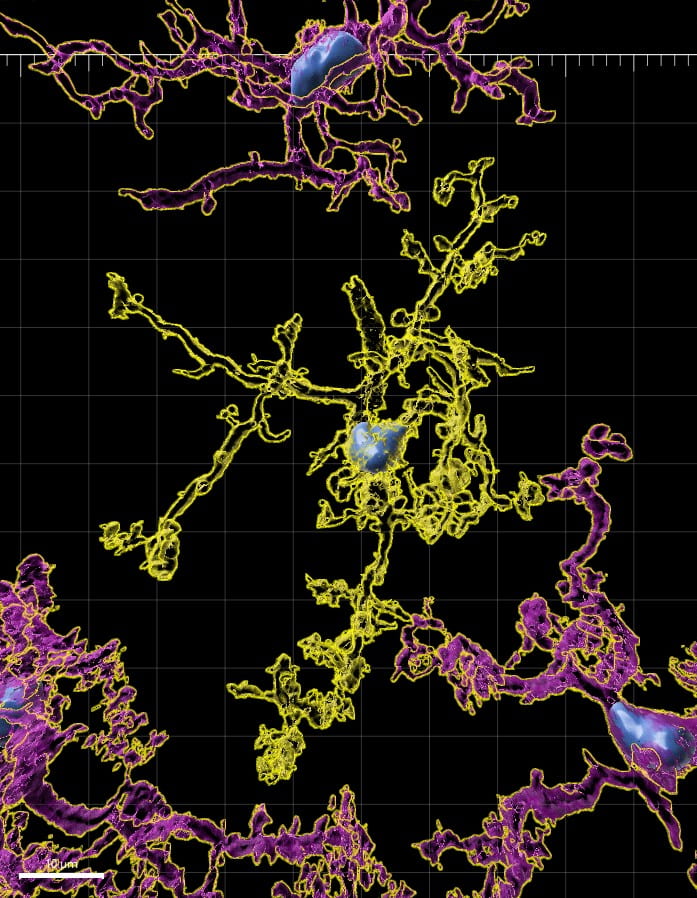
We and collaborators discovered a surprising new way to manipulate microglia, the brain’s primary immune cells which play key roles in neurological and psychiatric disease as well as in normal brain functions like learning. While microglia are well known to affect neural function, little is known about how neural activity affects these immune cells and brain immune function generally. Inspired by neural activity deficits we found in animal models of Alzheimer’s disease, we found that driving gamma oscillations (40Hz) mobilized microglia to remove amyloid beta, a protein whose aggregation is thought to initiate neurotoxic events in Alzheimer’s disease (Iaccarino*, Singer* et al. 2016). Specifically, driving gamma recruited microglia to increase engulfment of beta amyloid, resulting in 40% reduction in beta amyloid. We then discovered that these effects could be achieved with simple flickering lights and sounds at gamma frequency (similar to a fast strobe light and beeping). This simple and non-invasive sensory stimulation drove gamma oscillations in memory circuits, recruited immune cells, and rescued memory behavior (Singer et al. 2018, Martorell*, Paulson* et al. 2019). Interestingly, these sensory stimulation rescues prospective coding, or representations of future positions thought crucial for planning (Paulson et al. 2025). Using this sensory stimulation, we have discovered a rapid and transient cascade of intracellular and extracellular biochemical immune signals by which driving gamma recruits immune cells (Garza et al. 2020). Furthermore, different frequencies of stimulation cause opposite effects on immune signals (Prichard*, Garza* et al. 2023). Thus, we have developed a tool to rapidly increase or decrease genes and proteins that control brain immune function. Now we are using this flexible approach to manipulate neuroimmune function in other diseases.
Relevant Publications
- 40 Hz sensory stimulation enhances CA3-CA1 coordination and prospective coding during navigation in a mouse model of Alzheimer’s disease.
Paulson AL, Zhang L, Prichard AM, Singer AC (2025)
PNAS
https://doi/10.1073/pnas.2419364122
- Brain rhythms control microglial response and cytokine expression via NFκB signaling.
Prichard A+, Garza KM+, Shridhar A, He C, Bitfaran S, Wang Y, Goodson MC, Jaeger D, Wood LB++, Singer AC++ (2023)
Science Advances
https://doi.org/10.1126/sciadv.adf5672
- A feasibility trial of gamma sensory Flicker for patients with prodromal Alzheimer’s Disease. He Q, Colon-Motas K, Pybus A, Piendel L, Seppa J, Walker M, Manzanares C, Qui D, Miocinovic S, Wood L, Levey A, Lah J, Singer AC (2021)
Alzheimer’s & Dementia: TRCI
https://doi.org/10.1002/trc2.12178
Received press coverage.
- Gamma visual stimulation induces a neuroimmune signaling profile distinct from acute neuroinflammation. Garza K, Zhang L, Boron B, Attokaren M,Wood L++, Singer AC++ (2020)
Journal of Neuroscience
https://doi.org/10.1523/JNEUROSCI.1511-19.2019
Received press coverage. Resulted in patent applications.
- Multi-sensory gamma stimulation ameliorates Alzheimer’s-associated pathology and improves cognition. Martorell AJ*, AL Paulson*, H-J Suk, F Abdurrob, GT Drummond, W Guan, JZ Young, DN-W Kim, O Kritskiy, SJ Baker, V Mangena, SM Prince, EN Brown, KC Chung, ES Boyden, AC Singer,L-H Tsai
Cell
https://www.cell.com/cell/fulltext/S0092-8674(19)30163-1
Received press coverage. Resulted in multiple clinical trials.
- Non-invasive 40 Hz light flicker to reduce amyloid load and recruit microglia. Singer AC++, AJ Martorell, JM Douglas, F Abdurrob, M Attokaren, J Tipton, H Mathys, C Adaikkan, and L-H Tsai++ (2018)
Nature Protocols.
https://www.nature.com/articles/s41596-018-0021-x
- Gamma oscillations attenuate amyloid pathology and trigger a distinct microglia response in a mouse model of Alzheimer’s disease. Iaccarino HF*, Singer AC*, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, Mathys H, Seo J, Kritskiy O, Abdurrob F, Adaikkan C, Canter RG, Rueda R, Brown EN, Boyden ES, Tsai L-H (2016)
Nature.
doi: 10.1038/nature20587. PMID: 27929004.
Published with News and Views. Recommended on Faculty of 1000. Received press coverage.
- Principles of Designing Interpretable Optogenetic Behavior Experiments.
BD Allen*, Singer AC*, ES Boyden (2015)
Learning and Memory.
doi: 10.1101/lm.038026.114. PMID: 25787711.
Invited review.
Translation to Humans
Using non-invasive sensory stimulation, we are translating our results from mice to humans to determine if this stimulation reaches memory circuits and manipulates neuroimmune function in humans. We completed a feasibility trial of gamma sensory stimulation in patients with prodromal Alzheimer’s disease in collaboration with Drs. Allan Levey and Jim Lah at the Emory Brain Health Center (He et al. 2021). We found excellent safety, tolerance, and adherence to gamma sensory Flicker and preliminary evidence that this stimulation affects the human neuroimmune system. Furthermore, we have found that this stimulation entrains neural activity in human memory circuits using intracranial recordings in epilepsy patients and decreases pathological subclinical epileptiform activity (Blanpain et al. 2024). These discoveries reveal new ways to non-invasively manipulate the human neuroimmune system and non-invasively target memory circuits in humans.
Relevant Publications
- Multisensory Flicker Modulates Widespread Brain Networks and Reduces Interictal Epileptiform Discharges
Blanpain LT, Chen E, Park J., Walelign MY, Gross RE, Cabaniss BT, Willie JT++, Singer AC++ (2024)
Nature Communications
https://doi.org/10.1101/2023.03.14.23286691
- A feasibility trial of gamma sensory Flicker for patients with prodromal Alzheimer’s Disease. He Q, Colon-Motas K, Pybus A, Piendel L, Seppa J, Walker M, Manzanares C, Qui D, Miocinovic S, Wood L, Levey A, Lah J, Singer AC (2021)
Alzheimer’s & Dementia: TRCI
https://doi.org/10.1002/trc2.12178